References
1. “Emergent high-Tc ferroelectric ordering of strongly correlated and frustrated protons in a heteroepitaxial ice film”, Toshiki Sugimoto, Norihiro Aiga, Yuji Otsuki, Kazuya Watanabe and Yoshiyasu Matsumoto, Nature Physics, 12, 1063-1068 (2016).(Selected as News and Views)
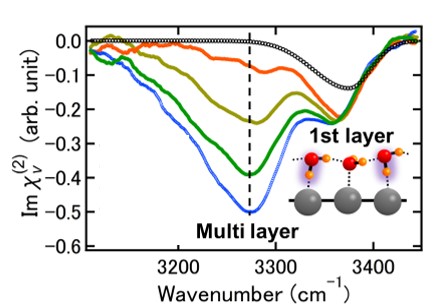
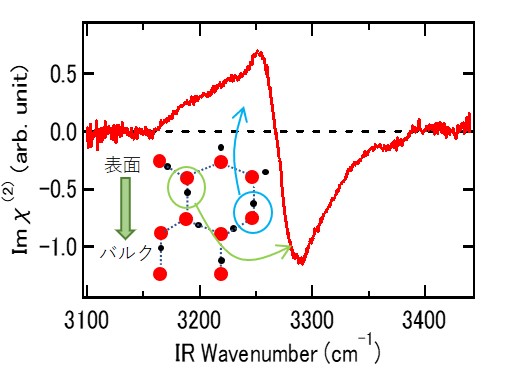
2. "Unveiling subsurface hydrogen-bond structure of hexagonal water ice", Y. Otsuki, T. Sugimoto, T. Ishiyama, A. Morita, K. Watanabe, and Y. Matsumoto Phys. Rev. B, 96, 115405 (2017).